What Best Describes a Covalent Bond
This type of bond may also be found in other chemical species such. - electronegativity difference between 01.
Covalent bonds occur through the interaction of neutral atoms.

. B two very electronegative atoms are covalently bound. Which of the following statements best describes a relatively polar covalent bond. Which of the following best describes a polar covalent bond.
Atoms will covalently bond with different atoms that allows you to benefit extra. An atom shares one electron with another for each covalent bond that it forms with another atom. Each oxygen atom needs two electrons and would form two covalent bonds.
- usually two of same elements bonded together. Covalent bonds can form between two nonmetal atoms. The atoms valence electrons combine to form a network of bonds.
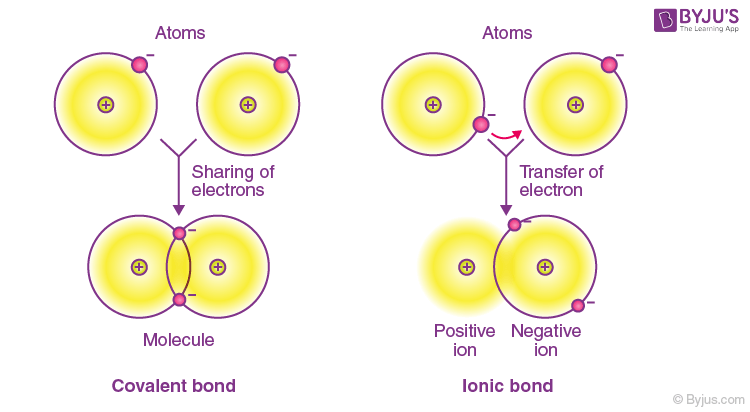
A pair of electrons shared between two atoms where each atom has donated one electron. It is formed when pairs of electrons are shared by atoms. A covalent bond may also be termed a molecular bond.
Electrons are transferred completely from one atom to another b. In contrast covalent bonds are quite weak and hence most compounds exist in the gaseous phase. Covalent bond is the sharing of electrons between atoms the electron pair is also known a shared pair.
Which of these statements best describes a dative covalent bond. Ionic bonds are the strongest type of chemical bond and therefore most compounds remain solid with very high melting points. The bonding is best described as covalent and because of the difference in electronegativity the bond is polar.
Covalent bonds can form between atoms of different elements. What statement best describes a social contract. A covalent bond happens when the positive nuclei from two different atoms are held together by their common attraction for the shared pair of electrons held between them.
Each atom is two electrons away from an octet of eight electrons. Two pairs of electrons shared between two atoms where each atom has donated one electron. A bond formed by the sharing of neutrons.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. A chemical bond in which no electrons are shared. A bond formed by the sharing of protons.
Electrons are shared but are pulled more strongly towardby one of the atoms d. Asked Sep 10 2016 in Chemistry by MagicCarpetRide. A pair of electrons shared between two atoms where one atom has donated two electrons.
A covalent bond is. Covalent bond is also known as chemical bond or a molecular bond. Which statement best describes covalent bonding.
Covalent bond is defined as the bond which is formed when sharing of electrons takes place between the atoms. Hydrogen and chlorine atoms are need only 1 electron to attain stable electronic configuration. What are the two type of covalent bonding.
How is the covalent bond formed answer. A covalent bond in chemistry is a chemical link between two atoms or ions in which the electron pairs are shared between them. - even distribution of chargeno dipole.
Atoms in a molecule. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ionsA covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
The atoms valence electrons are shared between the atoms. What is a nonpolar covalent bond. Non-polar covalent bonds form when two atoms share electrons unequally with one electron spending more time around one atom than another the molecule becomes slightly positive on one side and slightly negative on the other giving the molecule opposite charges on two sides.
All of the atoms electrons are shared between the atoms please help. Covalent bonds form between two nonmetal atoms with identical or relatively close electronegativity values. By sharing their outer most valence electrons atoms deserve to fill increase their outer electron.
A chemical bond in which four or more electrons are shared. Which statement correctly describes a covalent bond. Neither atom is strong.
Which of the following best describes covalent bonding. The binding arises from the electrostatic attraction of their nuclei for the same electrons. - electronegativity is 0-04.
Valence electrons are transferred from one atom to the other. - equal sharing of electrons. A It is a type of chemical bonding exhibited in compounds that contain positively and negatively charged ions.
Covalent bond in chemistry the interatomic linkage that results from the sharing of an electron pair between two atoms. - unequal sharing of electrons. Electrons in the bond are shared equally by the atoms.
So they share electron and form covalent bond. C two very electronegative atoms undergo ionic bonding. Answer Expert Verifiedquestionquestion mark A covalent bond is fashioned among non-metals which have comparable electronegativities.
Covalent bonds can form between atoms of the same element. This is usually formed between two non-metals. A bond formed by the sharing of electrons.
A bond formed by the gain of electrons. Identify which of the following statements best describes a covalent bond. Covalent bonds are strong bonds.
Covalent bonding occurs once pairs of electron are shared by atoms. A chemical bond that involves sharing a pair of electrons between. A bond formed by the loss of electrons.
Electrons are shared but the shared electrons came entirely from one of the bonded atoms c. The pair of electrons. A covalent bond is formed by equal sharing of electrons from both the participating atoms.
There would be two covalent bonds in each O₂ molecule. Electronegativity of covalent bonds is below than 17 because if the electronegativity is more than 17 the bond will be ionic and an ionic bond donates electron to the other. B It is a type of chemical bonding exhibited in compounds that.
Atoms that share pairs of electrons form molecules. Atoms will covalently shortcut with various other atoms in order to gain much more stability which is obtained by forming a complete electron shell. A a very electronegative atom and a weakly electronegative atom are covalently bound.

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Covalent Bond Definition Properties Examples Facts Britannica

Covalent Bond Definition Types And Examples

Which One Of The Following Best Describes A Job Cost Sheet In 2022 Cost Sheet Job Sheet

What Are Covalent Bonds And What Are They Also Known As Quora

Welcome To Learnapchemistry Com Ap Chemistry Chemical Science Ap Chem

Gcse Chemistry Covalent Bonding In A Water Molecule What Is The Structure Of A Water Molecule Water Molecule Covalent Bonding Water Molecule Structure

Covalent Bond Definition Types And Examples

Covalent Bond Definition Types And Examples

Design Everywhere Print Designs Inspiration Graphic Design Graphic Design Studios

Covalent Compounds Covalent Bond Properties Examples With Videos

Practice With Covalent Bonding Covalent Bonding Teaching Chemistry Science Classroom
What Is A Covalent Bond And How Is It Formed Quora

Atomic Interactions Molecular Geometry Covalent Bonding Interactive

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Which Of The Following Phrases Best Describes Process Focus In 2022 Process The Selection Focus

Ionic Vs Covalent Coloring Activity Chemistry Science Pdf Printable From Laurelsusanstudio On Teachersnotebook Com 3 Pages Chemia


Comments
Post a Comment